Is your business in need of Secure Software and Risk Consulting?
At TEKenable, our commitment to secure software development goes beyond industry standards. We adhere to ISO27001, ISO9001, and Software as a Medical Device standards ISO13485 and IEC62304 to ensure your company’s digital solutions are not only functional but fortified against potential risks.

Empowering your C-Suite: Secure Software & Risk Consulting for greater Cyber Resilience
Our developed software is ISO27001:2013 compliant and resilient against OWASP Top 10 attacks. DevSecOps integrates automated penetration testing and static code inspection. Third-party security assessments are common during customer acceptance testing.
Our credentials include CISSP, CISM, CSSLP, ISO certifications, and Cyber Essentials, reaffirming our cybersecurity dedication.
Secure Software Development
Security cannot be added to software after it has been built. At TEKenable we start with the end goal in mind – beginning with a clear understanding of the risks and needs of the software application and embedding security at each stage – from architecture, design, implementation and the testing process. We call this our Secure Software Development Life Cycle (SSDLC). The image below shows the steps.
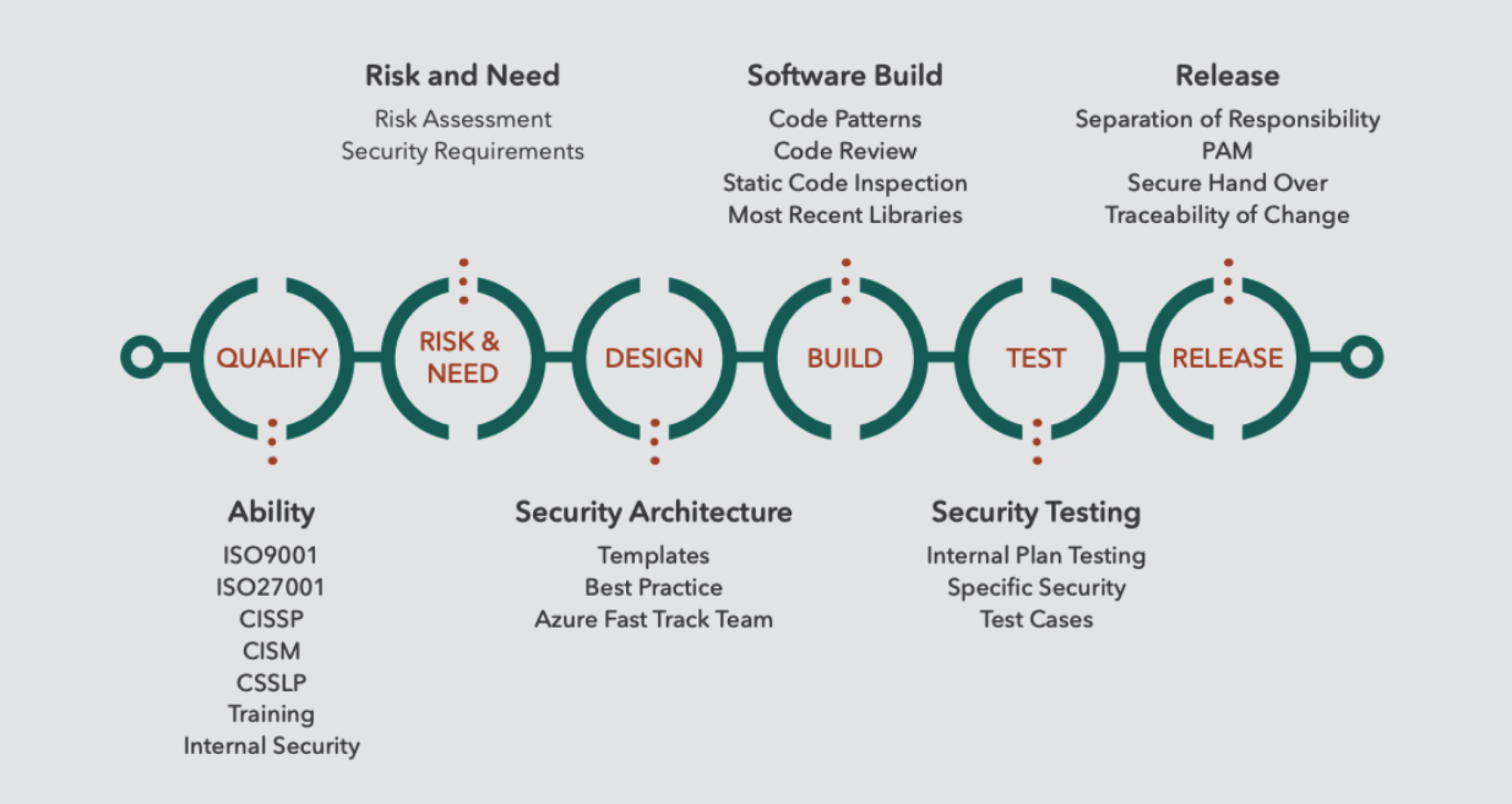
Risk and Cyber Security Consulting
We provide advice and guidance on a wide range of cyber security topics.
Our cyber security team are CISSP accredited (Certified Information Systems Security Professionals), with qualifications in CISM (Certified Information Security Management) and CSSLP (Certified Secure Software Lifecycle Professionals).
We also have specialist Azure Architects, DevSecOps Engineers and over three decades of practical real world experience delivering enterprise solutions in secure software development and risk consulting.
Medical Device Software
We specialize in healthcare secure software development and risk consulting, particularly for developing medical apps categorized as Software as a Medical Device (SaMD) as per medical device regulations. Our experts are well-versed in the Microsoft technology stack and other cutting-edge technologies. By adhering to the IEC 62304 software development process, we ensure our software applications align with ISO 13485 regulations. Our partner, Greenfinch Technology, is an Irish software development firm with a SaMD focus. We collaborate with medical firms in Ireland, the UK, and even the United States.
Ensuring FDA-CFR Compliance for Your Business
With the implementation of the 21 CFR Part 11 rule, the FDA has paved the way for regulated industries to optimise processes, cut costs, and accelerate turnaround time. This standardises the use of electronic records and signatures, enabling streamlined management of records and content. By minimising human errors, lowering operational expenses, and expediting pharmaceutical product launches, this rule offers significant benefits to businesses.
TEKenable’s Expertise in Validated Systems and Clinical Trials Support:
- Clinical Trials
- Stability Trials
- Adverse Event Reporting (pre and post market)
- Regulatory Licence Submission
FDA 21 CFR Part 11
FDA 21 CFR Part 11 is a pivotal section within the Code of Federal Regulations (CFR) framework, delineating the FDA’s directives for utilising electronic records and signatures. This regulation falls under Title 21 CFR, which encompasses guidelines for Pharmaceuticals and Medical Devices. Part 11 specifically addresses the realm of electronic records and electronic signatures in these industries.
Aligning with FDA Guidelines: Secure Software and Risk Consulting
Our Secure Software and Risk Consulting approach ensures compliance with FDA regulations regarding electronic records. Under 21 CFR Part 11, different software system classifications necessitate specific considerations during development:
For “closed” systems:
- Validation to ensure accuracy, reliability, and consistent performance
- Storage of records in accessible formats for FDA inspection
- Maintenance of electronic records equivalent to paper records
- Implementation of secure, time-stamped audit trails for operator actions
- Controlled access for authorised personnel
For “open” systems:
- Potential encryption for document confidentiality
- Use of digital signature standards for authenticity and integrity
TEKenable’s expertise in Secure Software Development empowers us to comprehensively address CFR Part 11 requirements, providing clients with the tools to meet FDA standards and ensure secure software and risk consulting excellence.
